Uses
This medication is used to treat a certain type of cancer of the colon (large intestine) or rectum. Panitumumab is a man-made protein (monoclonal antibody) that binds to a certain protein (epidermal growth factor receptor-EGFR). It works by slowing or stopping the growth of cancer cells.
How to use Vectibix Vial
This medication is given by injection into a vein by a health care professional. It is given as directed by your doctor, usually every 14 days. The dosage is based on your weight and response to treatment.
Infusion reactions may happen during the infusion of this drug. Tell your doctor right away if you have any symptoms of infusion reactions such as fever, chills, shortness of breath, or dizziness. Your doctor may slow down or stop your treatment for some time.
Side Effects
See also Warning and How to Use sections.
Diarrhea, nausea, vomiting, tiredness, constipation, abdominal pain, and growth of eyelashes may occur. If any of these effects last or get worse, tell your doctor or pharmacist promptly.
Remember that this medication has been prescribed because your doctor has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects.
Tell your doctor right away if you have any serious side effects, including: swelling ankles/feet, unusual weakness, irregular heartbeat, severe muscle spasms, mouth sores, signs of eye problems (such as eye redness/itching/irritation, watery eyes, vision changes), signs of lung disease (such as cough, shortness of breath), menstrual changes.
Prolonged and/or severe diarrhea may lead to dehydration and electrolyte imbalance. Tell your doctor right away if you develop any of the following: extreme thirst, decreased urination, dizziness, fainting.
Get medical help right away if you have any very serious side effects, including: seizures.
A very serious allergic reaction to this drug is rare. However, get medical help right away if you notice any symptoms of a serious allergic reaction, including: rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing.
This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist.
In the US -
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch.
In Canada - Call your doctor for medical advice about side effects. You may report side effects to Health Canada at 1-866-234-2345.
Warnings
Panitumumab has caused very serious skin reactions, which can sometimes lead to serious infections. Tell your doctor right away if you develop any signs of a skin reaction, including acne, mild rash/itching, warmth/redness/swelling of the skin (including around the nails), dry/flaking skin, or skin sores (especially with pus). Get medical help right away if you develop any signs of a serious infection (such as sepsis), including fever, fast heartbeat, mental/mood changes (such as confusion), or signs of kidney problems (such as change in the amount of urine).
Precautions
Before receiving panitumumab, tell your doctor or pharmacist if you are allergic to it; or if you have any other allergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details.
Before using this medication, tell your doctor or pharmacist your medical history, especially of: lung disease (such as pulmonary fibrosis, interstitial pneumonitis), low magnesium/calcium blood levels.
Sunlight may worsen any skin reactions that may occur while you are receiving this drug. Limit your time in the sun. Avoid tanning booths and sunlamps. Use sunscreen and wear protective clothing when outdoors.
Before having surgery, tell your doctor or dentist about all the products you use (including prescription drugs, nonprescription drugs, and herbal products).
Tell your doctor if you are pregnant or plan to become pregnant. You should not become pregnant while using panitumumab. Panitumumab may harm an unborn baby. Ask about reliable forms of birth control while using this medication and for 2 months after the last dose. If you become pregnant, talk to your doctor right away about the risks and benefits of this medication.
Based on information from related drugs, this medication may pass into breast milk. Breastfeeding is not recommended while using this medication and for 2 months after the last dose. Consult your doctor before breastfeeding.
Interactions
Drug interactions may change how your medications work or increase your risk for serious side effects. This document does not contain all possible drug interactions. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor's approval.
Overdose
If someone has overdosed and has serious symptoms such as passing out or trouble breathing, call 911. Otherwise, call a poison control center right away. US residents can call 1-800-222-1222. Canada residents can call 1-844-764-7669.
Lab and/or medical tests (such as magnesium/calcium levels) should be done before you start using this medication, while you are using it and for 8 weeks after completion of treatment. Keep all medical and lab appointments. Consult your doctor for more details.
It is important to get each dose of this medication as scheduled. If you miss a dose, ask your doctor or pharmacist right away for a new dosing schedule.
Not applicable. This medication is given in a clinic and will not be stored at home.
Images
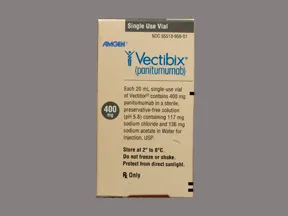
Vectibix 400 mg/20 mL (20 mg/mL) intravenous solution
Color: colorlessShape: Imprint:This medicine is a colorless, vial
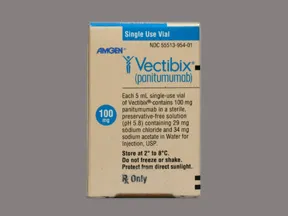
Vectibix 100 mg/5 mL (20 mg/mL) intravenous solution
Color: colorlessShape: Imprint:This medicine is a colorless, vial
Are you currently using Vectibix Vial?
This survey is being conducted by the WebMD marketing sciences department.
Selected from data included with permission and copyrighted by First Databank, Inc. This copyrighted material has been downloaded from a licensed data provider and is not for distribution, except as may be authorized by the applicable terms of use.
CONDITIONS OF USE: The information in this database is intended to supplement, not substitute for, the expertise and judgment of healthcare professionals. The information is not intended to cover all possible uses, directions, precautions, drug interactions or adverse effects, nor should it be construed to indicate that use of a particular drug is safe, appropriate or effective for you or anyone else. A healthcare professional should be consulted before taking any drug, changing any diet or commencing or discontinuing any course of treatment.