Uses
This medication is used to treat certain mental/mood disorders (such as schizophrenia, bipolar disorder). Ziprasidone belongs to a class of drugs known as atypical antipsychotics. It works by helping to restore the balance of certain natural substances in the brain.
How to use Ziprasidone Hcl
Read the Patient Information Leaflet if available from your pharmacist before you start taking ziprasidone and each time you get a refill. If you have any questions, ask your doctor or pharmacist.
Take this medication by mouth with food as directed by your doctor, usually twice daily. Swallow the capsules whole. Do not open, crush, or chew the capsules.
The dosage is based on your medical condition and response to treatment. To reduce your risk of side effects, your doctor may direct you to start this medication at a low dose and gradually increase your dose. Follow your doctor's instructions carefully.
Take this medication regularly to get the most benefit from it. To help you remember, take it at the same time each day. Keep taking this medication even if you feel well. Do not stop taking this medication without consulting your doctor.
Tell your doctor if your condition lasts or gets worse.
Side Effects
Drowsiness, dizziness, lightheadedness, weakness, nausea, vomiting, runny nose, and cough may occur. If any of these effects last or get worse, tell your doctor promptly.
Dizziness and lightheadedness can increase the risk of falling. Get up slowly when rising from a sitting or lying position.
Remember that this medication has been prescribed because your doctor has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects.
Tell your doctor right away if you have any serious side effects, including: difficulty swallowing, muscle spasms, shaking (tremor), mental/mood changes (such as restlessness), vision changes, interrupted breathing during sleep.
This drug may rarely make your blood sugar rise, which can cause or worsen diabetes. Tell your doctor right away if you have symptoms of high blood sugar such as increased thirst/urination. If you already have diabetes, check your blood sugar regularly as directed and share the results with your doctor. Your doctor may need to adjust your diabetes medication, exercise program, or diet.
This drug may also rarely cause significant weight gain and a rise in your blood cholesterol (or triglyceride) levels. These effects, along with diabetes, may increase your risk for developing heart disease. Discuss the risks and benefits of treatment with your doctor.
This drug may rarely cause a condition known as tardive dyskinesia. In some cases, this condition may be permanent. Tell your doctor right away if you develop any unusual/uncontrolled movements (especially of the face, mouth, tongue, arms, or legs).
This medication may increase a certain natural substance (prolactin) made by your body. For females, this increase in prolactin may result in unwanted breast milk, missed/stopped periods, or difficulty becoming pregnant. For males, it may result in decreased sexual ability, inability to produce sperm, or enlarged breasts. If you develop any of these symptoms, tell your doctor right away.
Get medical help right away if you have any very serious side effects, including: severe dizziness, fainting, seizures, signs of liver damage (such as nausea/vomiting that doesn't stop, loss of appetite, stomach/abdominal pain, yellowing eyes/skin).
This medication may rarely cause a very serious condition called neuroleptic malignant syndrome (NMS). Get medical help right away if you have any of the following symptoms: fever, muscle stiffness/pain/tenderness/weakness, severe tiredness, severe confusion, sweating, fast/irregular heartbeat, dark urine, signs of kidney problems (such as change in the amount of urine).
Rarely, males may have a painful or prolonged erection lasting 4 or more hours. If this occurs, stop using this drug and get medical help right away, or permanent problems could occur.
A very serious allergic reaction to this drug is rare. However, get medical help right away if you notice any symptoms of a serious allergic reaction, including: fever, swollen lymph nodes, rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing.
This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist.
In the US -
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch.
In Canada - Call your doctor for medical advice about side effects. You may report side effects to Health Canada at 1-866-234-2345.
Warnings
There may be a slightly increased risk of serious, possibly fatal side effects (such as stroke, heart failure, fast/irregular heartbeat, pneumonia) when this medication is used by older adults with dementia. This medication is not approved for the treatment of dementia-related behavior problems. Discuss the risks and benefits of this medication, as well as other effective and possibly safer treatments for dementia-related behavior problems, with the doctor.
If you are using ziprasidone in combination with other medication to treat depression, also carefully read the drug information for the other medication.
Precautions
Before taking ziprasidone, tell your doctor or pharmacist if you are allergic to it; or if you have any other allergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details.
Before using this medication, tell your doctor or pharmacist your medical history, especially of: dementia, seizures, low white blood cell count, difficulty swallowing, heart disease (such as coronary artery disease, irregular heartbeat), diabetes (including family history), obesity, breathing trouble during sleep (sleep apnea).
Ziprasidone may cause a condition that affects the heart rhythm (QT prolongation). QT prolongation can rarely cause serious (rarely fatal) fast/irregular heartbeat and other symptoms (such as severe dizziness, fainting) that need medical attention right away.
The risk of QT prolongation may be increased if you have certain medical conditions or are taking other drugs that may cause QT prolongation. Before using ziprasidone, tell your doctor or pharmacist of all the drugs you take and if you have any of the following conditions: certain heart problems (heart failure, recent heart attack, slow heartbeat, QT prolongation in the EKG), family history of certain heart problems (QT prolongation in the EKG, sudden cardiac death).
Low levels of potassium or magnesium in the blood may also increase your risk of QT prolongation. This risk may increase if you use certain drugs (such as diuretics/"water pills") or if you have conditions such as severe sweating, diarrhea, or vomiting. Talk to your doctor about using ziprasidone safely.
This drug may make you dizzy or drowsy. Alcohol or marijuana (cannabis) can make you more dizzy or drowsy. Do not drive, use machinery, or do anything that needs alertness until you can do it safely. Avoid alcoholic beverages. Talk to your doctor if you are using marijuana (cannabis).
Before having surgery, tell your doctor or dentist about all the products you use (including prescription drugs, nonprescription drugs, and herbal products).
This medication may make you sweat less, making you more likely to get heat stroke. Avoid doing things that may cause you to overheat, such as hard work or exercise in hot weather, or using hot tubs. When the weather is hot, drink a lot of fluids and dress lightly. If you overheat, quickly look for a place to cool down and rest. Get medical help right away if you have a fever that does not go away, mental/mood changes, headache, or dizziness.
Older adults may be more sensitive to the side effects of this drug, especially drowsiness, dizziness, lightheadedness, uncontrolled movements, and QT prolongation (see above). Drowsiness, dizziness, and lightheadedness can increase the risk of falling.
During pregnancy, this medication should be used only when clearly needed. Babies born to mothers who have used this drug during the last 3 months of pregnancy may rarely develop symptoms including muscle stiffness or shakiness, drowsiness, feeding/breathing difficulties, or constant crying. If you notice any of these symptoms in your newborn especially during their first month, tell the doctor right away.
Since untreated mental/mood problems (such as schizophrenia, bipolar disorder, depression) can be a serious condition, do not stop taking this medication unless directed by your doctor. If you are planning pregnancy, become pregnant, or think you may be pregnant, immediately discuss with your doctor the benefits and risks of using this medication during pregnancy.
It is unknown if this drug passes into breast milk. Because of the possible risk to the infant, breastfeeding is not recommended while using this drug. Consult your doctor before breastfeeding.
Interactions
Drug interactions may change how your medications work or increase your risk for serious side effects. This document does not contain all possible drug interactions. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor's approval.
Some products that may interact with this drug are: dolasetron, metoclopramide.
Many drugs besides ziprasidone may affect the heart rhythm (QT prolongation), including amiodarone, dofetilide, moxifloxacin, pimozide, procainamide, quinidine, sotalol, tacrolimus, thioridazine, among others.
Tell your doctor or pharmacist if you are taking other products that cause drowsiness such as opioid pain or cough relievers (such as codeine, hydrocodone), alcohol, marijuana (cannabis), drugs for sleep or anxiety (such as alprazolam, lorazepam, zolpidem), muscle relaxants (such as carisoprodol, cyclobenzaprine), or antihistamines (such as cetirizine, diphenhydramine).
Check the labels on all your medicines (such as allergy or cough-and-cold products) because they may contain ingredients that cause drowsiness. Ask your pharmacist about using those products safely.
Overdose
If someone has overdosed and has serious symptoms such as passing out or trouble breathing, call 911. Otherwise, call a poison control center right away. US residents can call 1-800-222-1222. Canada residents can call 1-844-764-7669. Symptoms of overdose may include: severe drowsiness, unusual/uncontrolled movements.
Do not share this medication with others.
Lab and/or medical tests (such as blood mineral levels, blood sugar, EKG) should be done while you are taking this medication. Keep all medical and lab appointments. Consult your doctor for more details.
If you miss a dose, take it as soon as you remember. If it is near the time of the next dose, skip the missed dose. Take your next dose at the regular time. Do not double the dose to catch up.
Store at room temperature away from light and moisture. Do not store in the bathroom. Keep all medications away from children and pets.
Do not flush medications down the toilet or pour them into a drain unless instructed to do so. Properly discard this product when it is expired or no longer needed. Consult your pharmacist or local waste disposal company.
Images
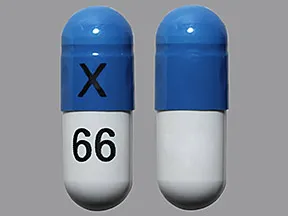
ziprasidone 80 mg capsule
Color: off-white,blueShape: oblongImprint: X 66This medicine is a off-white blue, oblong, capsule imprinted with "X" and "66".

ziprasidone 60 mg capsule
Color: off-whiteShape: oblongImprint: F 39This medicine is a off-white blue, oblong, capsule imprinted with "X" and "66".
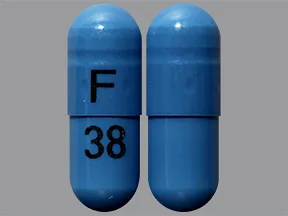
ziprasidone 40 mg capsule
Color: blueShape: oblongImprint: F 38This medicine is a off-white blue, oblong, capsule imprinted with "X" and "66".
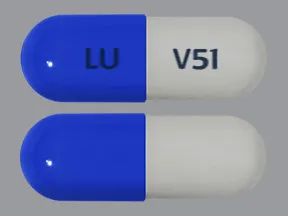
ziprasidone 20 mg capsule
Color: dark blue,whiteShape: oblongImprint: LU V51This medicine is a off-white blue, oblong, capsule imprinted with "X" and "66".

ziprasidone 40 mg capsule
Color: blueShape: oblongImprint: G 2002 G 2002This medicine is a off-white blue, oblong, capsule imprinted with "X" and "66".
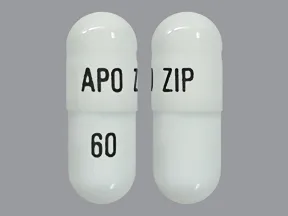
ziprasidone 60 mg capsule
Color: whiteShape: oblongImprint: APO ZIP 60This medicine is a off-white blue, oblong, capsule imprinted with "X" and "66".

ziprasidone 80 mg capsule
Color: dark blue,whiteShape: oblongImprint: LU V54This medicine is a off-white blue, oblong, capsule imprinted with "X" and "66".
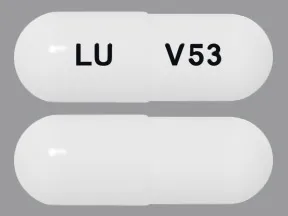
ziprasidone 60 mg capsule
Color: whiteShape: oblongImprint: LU V53This medicine is a off-white blue, oblong, capsule imprinted with "X" and "66".
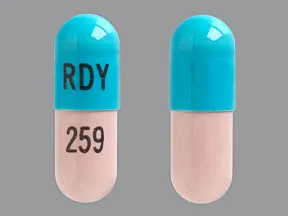
ziprasidone 80 mg capsule
Color: peach,light turquoiseShape: oblongImprint: RDY 259This medicine is a off-white blue, oblong, capsule imprinted with "X" and "66".

ziprasidone 20 mg capsule
Color: white,purpleShape: oblongImprint: APO ZIP 20This medicine is a off-white blue, oblong, capsule imprinted with "X" and "66".

ziprasidone 80 mg capsule
Color: white,blueShape: oblongImprint: G 2004 G 2004This medicine is a off-white blue, oblong, capsule imprinted with "X" and "66".

ziprasidone 60 mg capsule
Color: whiteShape: oblongImprint: G 2003 G 2003This medicine is a off-white blue, oblong, capsule imprinted with "X" and "66".

ziprasidone 40 mg capsule
Color: dark blueShape: oblongImprint: LU V52This medicine is a off-white blue, oblong, capsule imprinted with "X" and "66".
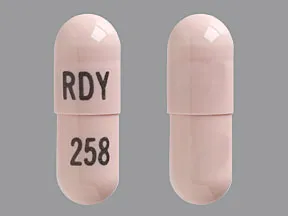
ziprasidone 60 mg capsule
Color: peachShape: oblongImprint: RDY 258This medicine is a off-white blue, oblong, capsule imprinted with "X" and "66".

ziprasidone 40 mg capsule
Color: purpleShape: oblongImprint: APO ZIP 40This medicine is a off-white blue, oblong, capsule imprinted with "X" and "66".

ziprasidone 40 mg capsule
Color: light turquoise,lavenderShape: oblongImprint: RDY 257This medicine is a off-white blue, oblong, capsule imprinted with "X" and "66".
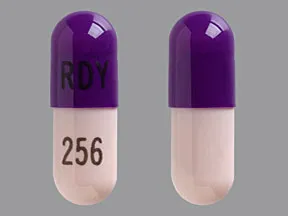
ziprasidone 20 mg capsule
Color: peach,lavenderShape: oblongImprint: RDY 256This medicine is a off-white blue, oblong, capsule imprinted with "X" and "66".
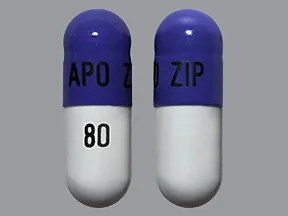
ziprasidone 80 mg capsule
Color: white,purpleShape: oblongImprint: APO ZIP 80This medicine is a off-white blue, oblong, capsule imprinted with "X" and "66".

ziprasidone 20 mg capsule
Color: white,blueShape: oblongImprint: G 2001 G 2001This medicine is a off-white blue, oblong, capsule imprinted with "X" and "66".
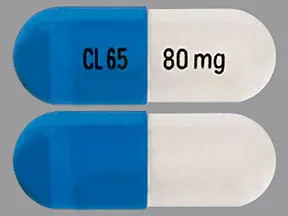
ziprasidone 80 mg capsule
Color: white,blueShape: oblongImprint: CL65 80 mgThis medicine is a off-white blue, oblong, capsule imprinted with "X" and "66".
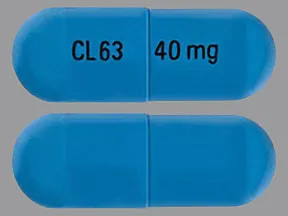
ziprasidone 40 mg capsule
Color: blueShape: oblongImprint: CL63 40 mgThis medicine is a off-white blue, oblong, capsule imprinted with "X" and "66".

ziprasidone 20 mg capsule
Color: white,blueShape: oblongImprint: CL 62 20 mgThis medicine is a off-white blue, oblong, capsule imprinted with "X" and "66".

ziprasidone 20 mg capsule
Color: off-white,blueShape: oblongImprint: F 26This medicine is a off-white blue, oblong, capsule imprinted with "X" and "66".
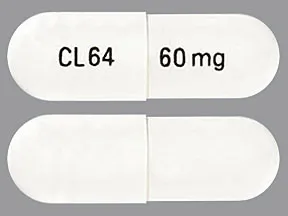
ziprasidone 60 mg capsule
Color: whiteShape: oblongImprint: CL64 60 mgThis medicine is a off-white blue, oblong, capsule imprinted with "X" and "66".
Are you currently using Ziprasidone Hcl?
This survey is being conducted by the WebMD marketing sciences department.
Selected from data included with permission and copyrighted by First Databank, Inc. This copyrighted material has been downloaded from a licensed data provider and is not for distribution, except as may be authorized by the applicable terms of use.
CONDITIONS OF USE: The information in this database is intended to supplement, not substitute for, the expertise and judgment of healthcare professionals. The information is not intended to cover all possible uses, directions, precautions, drug interactions or adverse effects, nor should it be construed to indicate that use of a particular drug is safe, appropriate or effective for you or anyone else. A healthcare professional should be consulted before taking any drug, changing any diet or commencing or discontinuing any course of treatment.